Non-invasive, non-destructive authentication of packaged medicines
Safer Medicines, Better World: In 2013, the United Nations Human Rights Council (UNHCR) adopted a resolution (A/HRC/RES12/24) on access to medicines that stressed “the responsibility of States to ensure access to all, without discrimination, of medicines, in particular essential medicines, that are affordable, safe, effective and of good quality”.
Current methods for authenticating medicines rely on optical spectroscopy or wet chemistry and for best effect require that the pill be removed from the packaging prior to testing, hence only a small number of packets from a suspect consignment can be tested for economic reasons.
The Medicines Authentication Unit within the Humanitarian Technologies Lab (HTL), Department of Informatics, King’s College London, has developed an authentication approach based on radiofrequency spectroscopy. The medicines can be examined without being removed from the packaging, making it possible to test all packets in a suspect consignment without the economic cost associated with current methods. Another advantage is that it allows detection of counterfeit as well as substandard and degraded drugs. The method is designed for solids and is suited for pills, powders and suspensions.
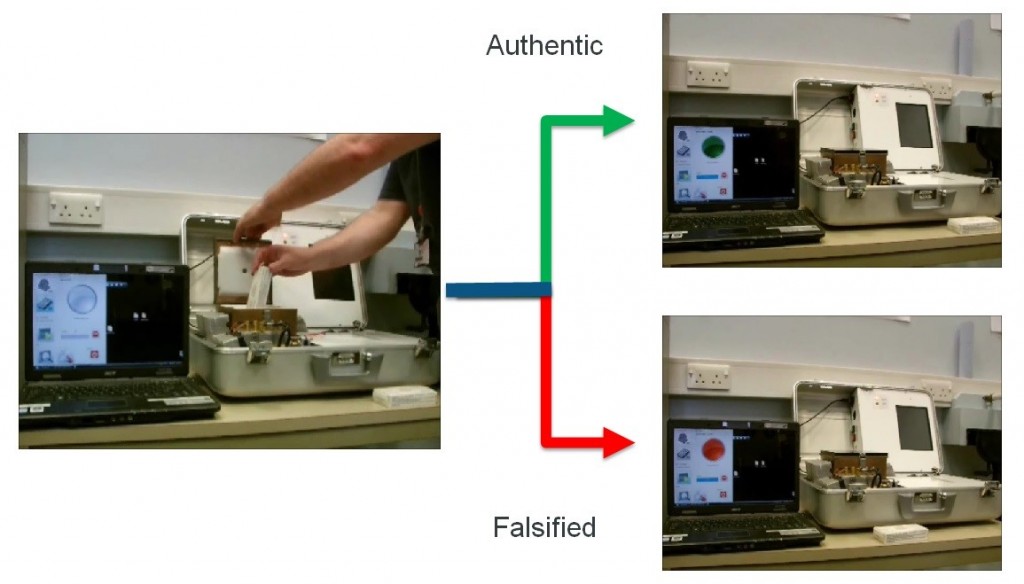
Portable technology demonstrator authenticating packaged medicines
Advantages
- Non-invasive and non-destructive
- Can be used on packaged medicines and consignments in parcels
- Can detect counterfeit as well as substandard and degraded drugs
- Can determine material proprties such as form, physical processing and other effects e.g. ageing
- Adaptable to mobile operation
- Simple Yes/ No response
Technical Background
Quadrupole Resonance Spectroscopy (QR) is a radiofrequency-based spectroscopic technique that returns signals from solid materials such as pharmaceutical active ingredients specific to the chemistry of the material under investigation. These signals are characteristic of not just to the material present, but also the amount of material present, the form (e.g. polymorph, ratio of stereoisomers) physical processing (e.g. loose powder, pressed pill) and even effects due to ageing. As the technology is radiofrequency-based, it is able to work through non-metallic packaging, eliminating the need to remove the medicines from their packaging prior to analysis. Not all molecules contain QR-sensitive elements, but, as both nitrogen-14 and Chlorine-35 are QR-sensitive, most active pharmaceutical ingredients do. There are no consumables required for quadrupole resonance, simply an electrical supply that can come from rechargeable batteries. Also, unlike with laser-based methods, no special precautions are required for working with radiofrequency electromagnetic radiation, making the technology ideal for adaptation for mobile operation.
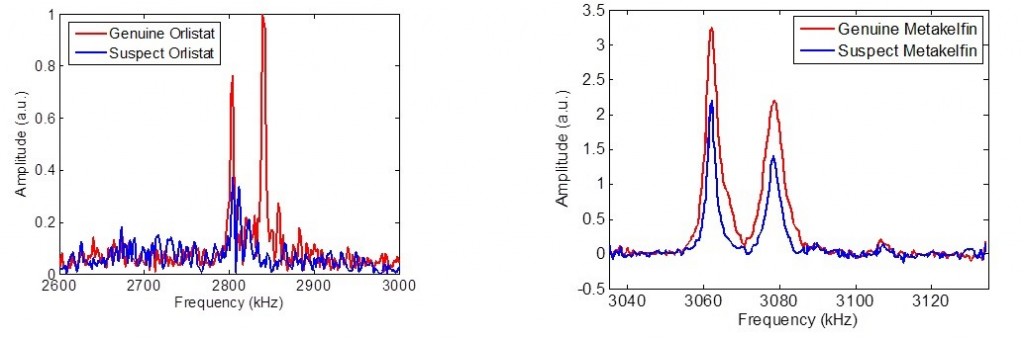
Two cases of medicines falsification successfully identified using QR, illustrating that the method is both qualitative and quantitative. Orlistat is a slimming aid, and metakelfin an anti-malarial. The metakelfin case shows that even when the correct material is present, if it is not present in the correct amount QR still spots the deception.

Trialling a proof-of-concept device at a postal sorting office in Warsaw.
References
Barras, K Althoefer, M. D. Rowe, I. J. P. Poplett and J. A. S. Smith, “The Emerging Field of Nuclear Qudrupole Resonance-Based Medicines Authentication”, Appl. Magn. Reson. 2012, 43, 511 – 529.
Barras, S. Katsura, H. Sato-Akaba, H. Itozaki, G. Kyriakidou, M. D. Rowe, K. Althoefer and J. A. S. Smith, “Variable-Pitch Rectangular Cross-section Radiofrequency Coils for the Nitrogen-14 Nuclear Quadrupole Resonance Investigation of Sealed Medicines Packets”, Analytical Chemistry, 2012, 84, 8970–8972.
Barras, D. Murnane, K. Althoefer, S. Assi, M. D. Rowe, I. Poplett, G. Kyriakidou and J. A. S. Smith, “Nitrogen-14 Nuclear Quadrupole Resonance Spectroscopy: a promising new analytical methodology for medicines authentication and counterfeit antimalarial analysis”, Analytical Chemistry, 2013, 84, 2746 – 2753.