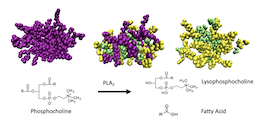
In this manuscript, we elucidate the underlying molecular scale mechanisms which drive the changes in the dynamic and structural properties of phosphatidylcholine micelles as they are digested by the phospholipase A2 (PLA2) enzyme. Specifically, we have investigated micelles consisting of dihexanoylphosphatidylcholine (2C6PC) which have potential as drug delivery vehicles for hydrophobic anticancer agents. 2C6PC affords the intriguing property that the products of its digestion by PLA2 (1-hexanoyl-lysophosphocholine (C6LYSO) and hexanoic acid (C6FA)) are both cell membrane permeability enhancers. Given that PLA2 is overexpressed in tumour cells, then the targeting of 2C6PC micelles to tumour sites will lead naturally to their digestion, and this will result in an enhanced delivery of their drug cargo across the tumour cell membranes. However, little is known about the behaviour of these products during and after the digestion of 2C6PC. In this work, we have used a combination of static and time-resolved small angle neutron scattering and all-atom molecular dynamics simulations to characterize the structure of the parent 2C6PC micelles and obtain a detailed understanding of the behaviour of its degradation products.
The results of our work show that stable micelles are formed by 2C6PC and the products as the degradation progresses. Also, by combining our molecular dynamics simulations and neutron scattering experiments we determine that the micelles formed by 2C6PC are best represented by a triaxial model where previously other models that had been used to describe these micelles had provided significantly different dimensions and aggregation numbers. Additionally, our results allow us to demonstrate the driving forces of the micellization of 2C6PC and its degradation products. Specifically, we show that the presence of lipid molecules with two hydrocarbon tails (e.g. 2C6PC) are required to stabilise the micelles. During the degradation process, we found that the fatty acid product, C6FA, forms the core of the micelle while C6LYSO coats the surface of this core. Thus, as the relative amount of C6LYSO to 2C6PC increases, the micelles become more dynamic but remain intact. Finally, we found that, after total degradation of 2C6PC, the C6LYSO/C6FA mixed micelle that is formed retains a hydrophobic core which could be quite important in their ability to deliver the drugs to and into tumour cells.
Full reference: The impact of lipid digestion on the dynamic and structural properties of micelles, Demi L. Pink, Fabrizia Foglia, David J. Barlow, M. Jayne Lawrence & Christian D. Lorenz. Small (2021) doi:10.1002/smll.202004761.