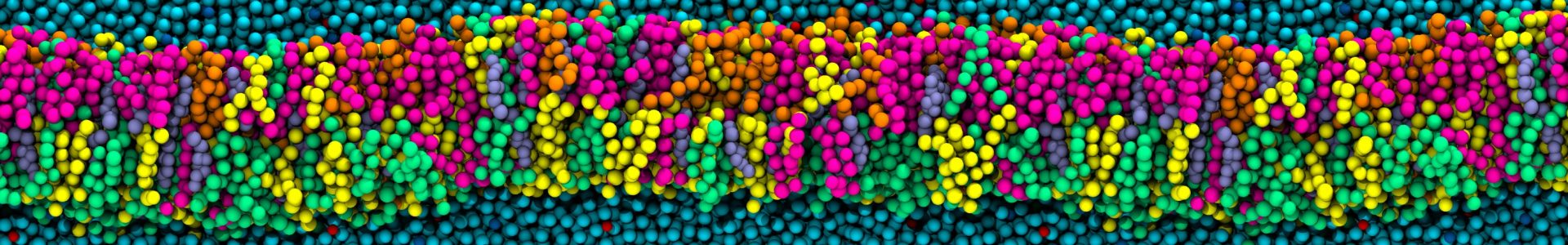
In our most recent paper in collaboration with James Mason, David Phoenix & J. Mark Sutton, we have carried out a multi-disciplinary investigation of the synergistic effects of the antimicrobial peptides, temporin B & temporin L. The temporins comprise a very well-studied family of AMPs that now number more than 130 peptides and whose members have been extensively evaluated and engineered to gain superior antibacterial activity. Temporin L is a broad- spectrum AMP, with potent bactericidal activity, identified, along with nine further temporins, in the European red frog Rana temporaria. Synergy against Gram-negative species has been described between temporin L and each of temporin A and temporin B which, individually, have only weak activity. Temporin L was shown to disrupt homo-oligomerization of both temporin A and temporin B, behaviour that would enhance their translocation across the outer membrane and access the bacterial plasma membrane, the presumed site of their membrane disruptive activity.
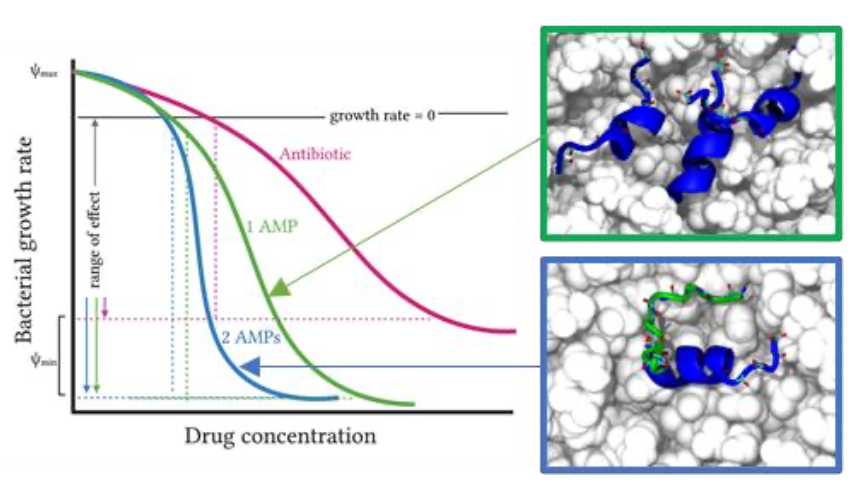
Here we use two time-resolved biophysical methods, molecular dynamics (MD) simulations and patch-clamp, to examine how a combination of temporin B and temporin L inserts into and disrupts models of the Gram-positive plasma membrane. We use this understanding to explain how the less potent temporin B can influence the cooperativity of the dose dependent bactericidal activity of temporin L against methicillin resistant S. aureus, which is in-turn compared with that of existing, clinically relevant antibiotics. Together, this provides a mechanistic perspective of how AMPs from the same organism may combine to enhance the pharmacodynamic profile and consequently reduce the risk of resistance to the innate immune response emerging.
Full reference: Temporin B forms hetero-oligomers with temporin L, modifies its membrane activity, and increases the cooperativity of its antibacterial pharmacodynamic profile. Philip M. Ferguson, Maria Clarke, Giorgia Manzo, Charlotte K. Hinds, Melanie Clifford, J. Mark Sutton, Christian D. Lorenz, David A. Pheonix and A. James Mason, Biochemistry (2022), doi: 10.1021/acs.biochem.1c00762