The 2016 Review on Antimicrobial Resistance (AMR) predicts that, unless action is taken, around 10 million deaths per year will be attributable to AMR by the year 2050. The pipeline of new antibiotics is limited, hence the potential of numerous alternatives to antibiotics – “noncompound approaches (i.e. products other than classic antibacterial agents) that target bacteria or approaches that target the host” – is being actively investigated. Commissioned by the Wellcome Trust, a pipeline portfolio review of alternatives to antibiotics recommends “strong support for funding while monitoring for breakthrough insights regarding systemic therapy” for a tier of approaches that include AMPs. The review presents the prevailing wisdom that AMPs are unsuited for systemic administration as they are poorly tolerated in animal models and susceptible to degradation. This substantially limits the scope of infection settings that are tractable to AMPs and hence their future development. There is an urgent need therefore to identify AMPs that are sufficiently potent against antibiotic resistant bacteria and well tolerated in vivo such that they are effective when delivered intravenously.
AMPs are a well-studied subset of a group of peptides that contribute to innate immunity, in a diverse range of organisms, through direct antimicrobial action and/or host defence regulation. Identified in the Winter Flounder, Pleuronectes americanus, pleurocidin is a potent AMP with broad spectrum anti-bacterial activity that seemingly belongs in the class of AMPs that acts by damaging the plasma membrane, with activity that is dependent on their ability to adopt an amphipathic α-helix conformation. Pleurocidin has high potency, at least against Gram-negative bacteria such as Escherichia coli, which is linked to its ability to cross the bacterial plasma membrane and penetrate within bacteria to attack intracellular targets. Importantly, the ordered α-helix conformation that pleurocidin adopts in many membrane mimics or models is less apparent in those models that most closely represent a Gram-negative bacterial cytoplasmic membrane. The increased conformational flexibility, detected when pleurocidin binds to such membranes, affords greater ability to penetrate into the hydrophobic core of the lipid bilayer, a property that we infer is critical to the potency associated with its intracellular penetration.
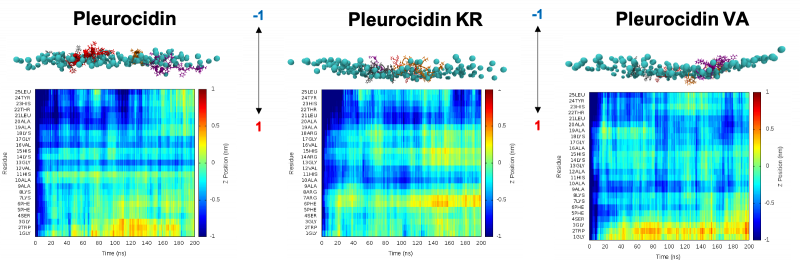
Strategies that increase the conformational flexibility of pleurocidin, when binding to model membranes, are likely to substantially alter its biological properties. We have previously identified that conformational flexibility is a peculiar property of pleurocidin when binding to models of bacterial plasma membranes. In our most recent study in collaboration with Dr A. James Mason (KCL, Institute of Pharmaceutical Science) and Dr J. Mark Sutton (Public Health England) this is shown to increase in two new analogues, (D)-pleurocidin-KR and (D)-pleurocidin-VA, but only in one, (D)-pleurocidin-KR, is this linked to increased antibacterial potency. We have used molecular dynamics simulations to gain an atomistic level of detail of the mechanism of action of these two analogues. We have found that the increased hydrogen bonding capability of the new analogue leads to greater membrane damage (and potentially a reduced ability to translocate). This new mechanism of action is more robust to changes in the bacterial metabolic strategy. Additionally, we have shown that analogues of pleurocidin, in particular (D)-pleurocidin-KR, are potent bactericidal AMPs which can be delivered intravenously to treat bacterial lung infections without triggering the release of pro-inflammatory cytokines or stimulating recruitment of innate immune cells in the mouse model. The modification strategy ensures that in some cases a 16-fold improvement in potency is observed for (D)-pleurocidin-KR over pleurocidin.
Full reference: “A pleurocidin analogue with greater conformational flexibility, enhanced antimicrobial potency and in vivo therapeutic efficacy“, Giorgia Manzo, Charlotte K. Hind, Philip M. Ferguson, Richard T. Amison, Alice C. Hodgson-Casson, Katarzyna A. Ciazynska, Bethany J. Weller, Maria Clarke, Carolyn Lam, Rico C. H. Man, Blaze G. O’ Shaughnessy, Melanie Clifford, Tam T. Bui, Alex F. Drake, R. Andrew Atkinson, Jenny K. W. Lam, Simon C. Pitchford, Clive P. Page, David A. Phoenix, Christian D. Lorenz, J. Mark Sutton & A. James Mason, Communications Biology (2020) 3, 697.