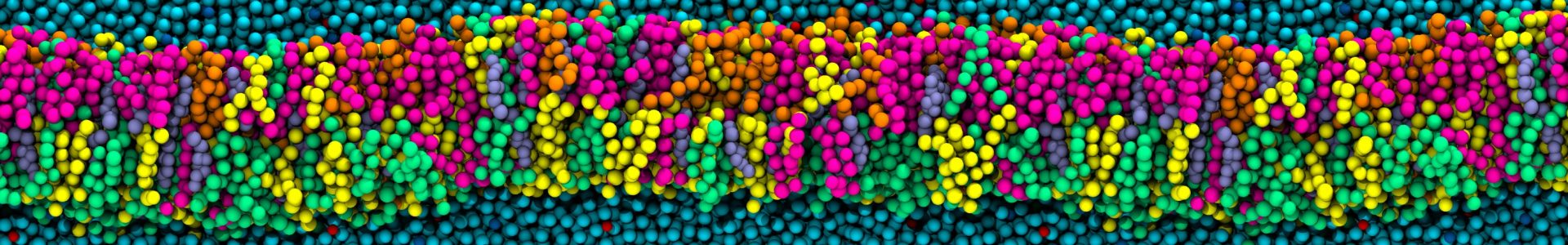
In this work, we elucidate the pH-directed self-assembly of Tetronic 304 (T304), a four-arm block copolymer. T304, as the smallest member of the Tetronics family, acts as an ideal testing ground to probe the underlying mechanisms that determine the self-assembly behaviour of this important class of polymers, which are of interest for use in drug delivery systems and molecular sensors. While a variety of experimental investigations have assessed T304’s uses in different pharmaceutical and therapeutic applications, a fundamental understanding of its pH-dir 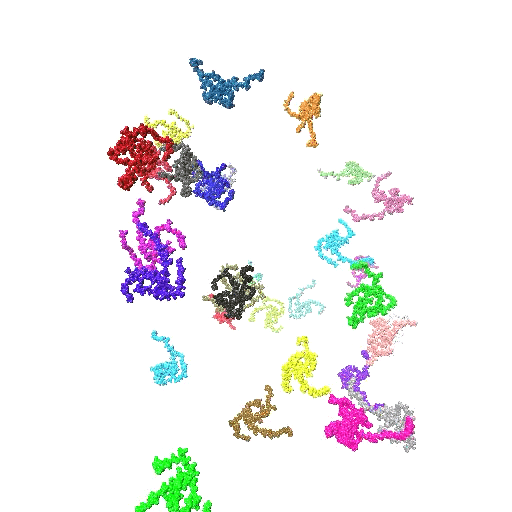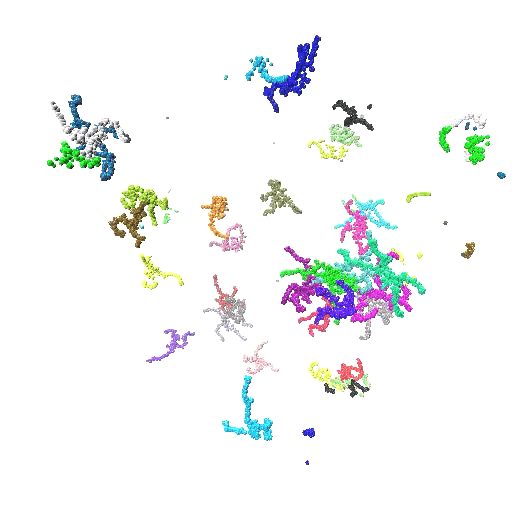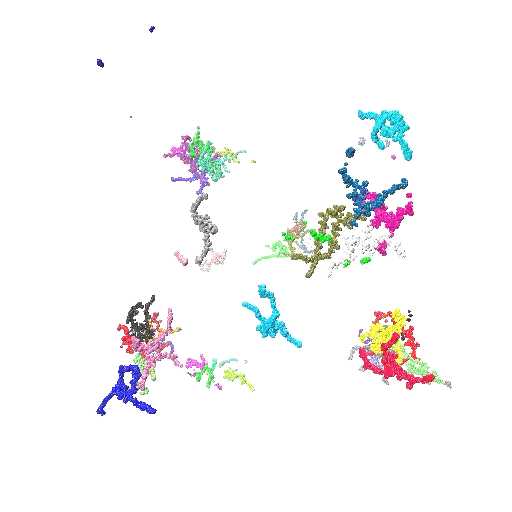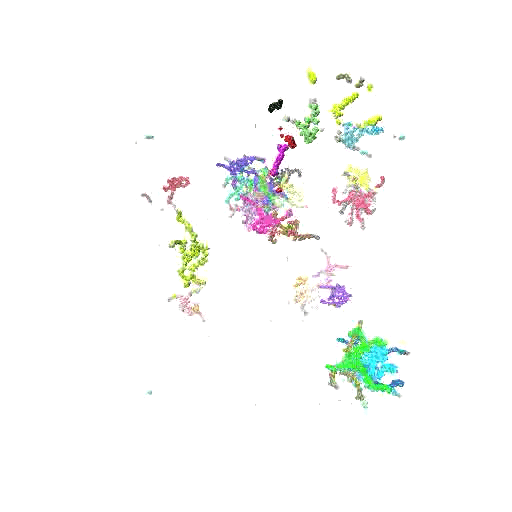 ected self-assembly is lacking. Furthermore, previously no atomistic simulations have been reported for the entire class of Tetronics. Also, we fully integrate scattering experiments and all-atom molecular dynamics simulations to study the pH-directed self-assembly of T304 for the first time. In doing so, we show that an internal conformation change in T304 is driven by pH, which greatly affects its subsequent interactions with water and in turn, controls its self-assembly behaviour.
ected self-assembly is lacking. Furthermore, previously no atomistic simulations have been reported for the entire class of Tetronics. Also, we fully integrate scattering experiments and all-atom molecular dynamics simulations to study the pH-directed self-assembly of T304 for the first time. In doing so, we show that an internal conformation change in T304 is driven by pH, which greatly affects its subsequent interactions with water and in turn, controls its self-assembly behaviour.
The importance of this work is that it:
- Provides a novel atomistic scale description of the pH-directed self-assembly of T304. Our simulations show the internal conformation of T304 is highly dependent on pH. We show that this phenomenon is due to the prevalence (at basic pH) of intramolecular hydrogen bonding, which in turn impacts the interactions of the central ethylene diamine group with water and the subsequent self-assembly behaviour. We show that interactions with the aqueous environment are an important factor to consider when rationalising self-assembly behaviour, rather than the typical arguments based entirely on mutual charge repulsion. As a variety of star and dendritic polymers contain ethylene diamine groups as central unit, this result provides insight that is significant in understanding the pH-directed behaviour of those polymers as well.
- Provides a combined experimental and atomistic description of the structure and solvation of T304 clusters. Our scattering experiments and atomistic simulations show that T304 self-assembly is greatly influenced by environmental pH, providing the first such study of this class of polymer. By using a series of simulations of the polymers at different protonation states, we have been able to describe the protonation state of the molecules at the different pHs we had studied experimentally. Additionally, we have used the results of our simulations to inform the choice of the model we used to fit our scattering experiments. In doing so, we develop an intrinsic core-shell interface (ISCI) model to analyse the intrinsic structure of T304 clusters and their interfacial water shell observed in our simulations, which provides interpretation of our experimental findings with unprecedented detail.
Full reference: Understanding the pH-Directed Self-Assembly of a Four-Arm Block Copolymer, Robert M Ziolek, Jasmin Omar, Wenjing Hu, Lionel Porcar, Gustavo Gonzalez-Gaitano, Cecile A. Dreiss & Christian D. Lorenz, Macromolecules (2020) 53 (24), 11065-11076