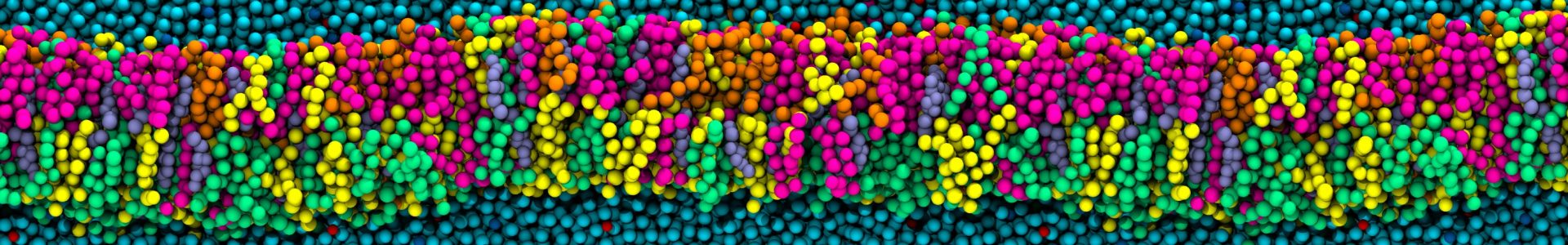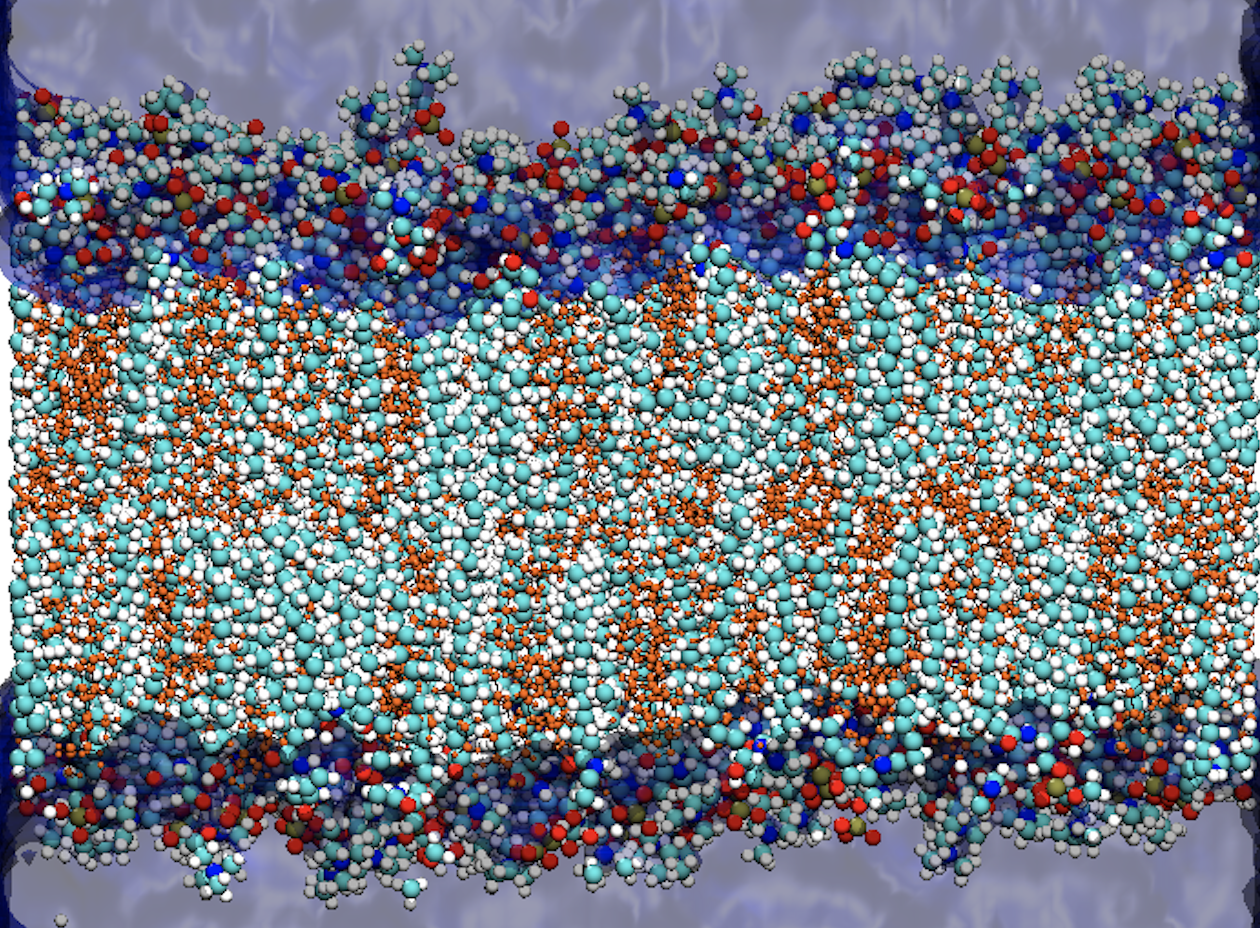
Sphingomyelin and cholesterol are prominent lipids in the plasma membrane of animal cells. In this manuscript, we have combined X-ray scattering experiments, performed by our collarborator Prof. Peter Quinn (KCL, Institute of Pharmaceutical Science), and all-atom molecular dynamics to investigate the bilayer structures observed within an equimolar mixture of palmitoylsphingomyelin and cholesterol. In doing so, we present vital insight into how specific molecular interactions between sphingomyelin and cholesterol molecules result in distinct conformations of the pair of interacting molecules and thereby determine the properties of the local lipid environment. The combination of small- and wide-angle X-ray scattering, along with molecular dynamics simulations, have allowed us to gain a unique perspective on how the molecular scale interactions in an equimolar mixture of palmitoylsphingomyelin (PSM) and cholesterol, at biologically-relevant temperatures (37 °C), govern the properties of the local lipid environment. Via a state-of-the-art Hidden Markov Model (HMM) and a novel unsupervised clustering algorithm, we are able to provide a detailed description of the structural properties of the lipids in the bilayer, and the interactions between them. This analysis has allowed us to provide the following important results:
- A detailed description of the molecular properties of the lipid molecules within the two bilayer structures observed in the X-ray scattering experiments. From small- and wide-angle X-ray scattering measurements, we have identified the presence of two distinct bilayer structures (one thicker, one thinner) in equimolar mixtures of PSM and cholesterol. The HMM allowed us to determine that the PSM molecules in the thicker membrane are characterized by more ordered, more extended and less interdigitated hydrocarbon tails compared to those of the PSM molecules in the thinner membrane. Intermolecular hydrogen bonds further distinguish the two bilayer structures, and we observe the disruption of a sphingomyelin intermolecular hydrogen bond network induced by the proximity of cholesterol.
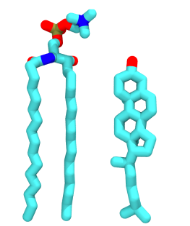
- The asymmetry of phospholipids is important in driving their interactions with cholesterol. From the application of the HMM to analyse our simulations, we found that the face of a cholesterol molecules with which a PSM molecule interacts impacts the physical properties of the PSM molecule, which has been reported previously. On the other hand, from a clustering of the interatomic distances of PSM-cholesterol pairs, we found that the face of a PSM molecule with which a cholesterol molecule interacts impacts the physical properties of the cholesterol molecule. Further, we found that the mutual orientation of interacting cholesterol and PSM molecules affects the physical properties of both molecules, and entirely determines which types of hydrogen bond are able to form between the pair of molecules. To the best of our knowledge, this is the first time the asymmetry of PSM, or of any phospholipid, has been shown to be important in driving its interactions with cholesterol.
- There are two mechanisms by which a PSM molecule shields a neighboring cholesterol molecule from the surrounding aqueous environment. We have applied an unsupervised clustering algorithm in order to characterize, for the first time, the most commonly found conformations formed between a pair of interacting PSM and cholesterol molecules. As a result, we are able to provide a new understanding of the Umbrella Model, which explains how phospholipids may shield cholesterol from water. Finally, we have discerned an interaction with the cholesterol molecules that can only be formed by sphingomyelin molecules, which may be the driving force for cholesterol to preferentially mix with sphingomyelin over glycerolipids.
Full reference: “Two coexisting membrane structures are defined by lateral and transbilayer interactions between sphingomyelin and cholesterol,” Paul Smith, Peter J. Quinn & Christian D. Lorenz, Langmuir (2020) 36 (33), 9786-9799.