Surfactant-covered aqueous interfaces are ubiquitous in everyday life. These interfaces play a key role in various commercial products, where surfactants adsorb onto the surface of bubbles or oil droplets to stabilize foams and emulsions. Examples of such foams and emulsions are commonly found in pharmaceuticals, detergents, cosmetics and lubricants. In our recent paper entitled “Structure and dynamics of nanoconfined water between surfactant monolayers“, we provide a detailed description of a nanoscale water layer confined between cetrimonium bromide (CTAB) monolayers.
Rob, Franca and Chris, in collaboration with Prof. Mesfin Tsige and Prof. Ali Dhinojwala (The University of Akron, USA), used atomistic molecular dynamics simulations to study the structure (density, orientation and coordination) and mobility of the confined water layer. This showed that small differences in the area per surfactant of the monolayers impart a clear effect on the water layer confined by the monolayers. As the area per surfactant within a monolayer decreases, the mobility of the interfacial water molecules decreases in response, with more water found “trapped behind” the CTAB headgroups. We also demonstrate the effects of competition between headgroups hinder the ordering of water at the interface. When two monolayers with different areas per surfactant are used to confine a nanoscale water layer, we observe the emergence of non-centrosymmetry, which has recently been observed experimentally.
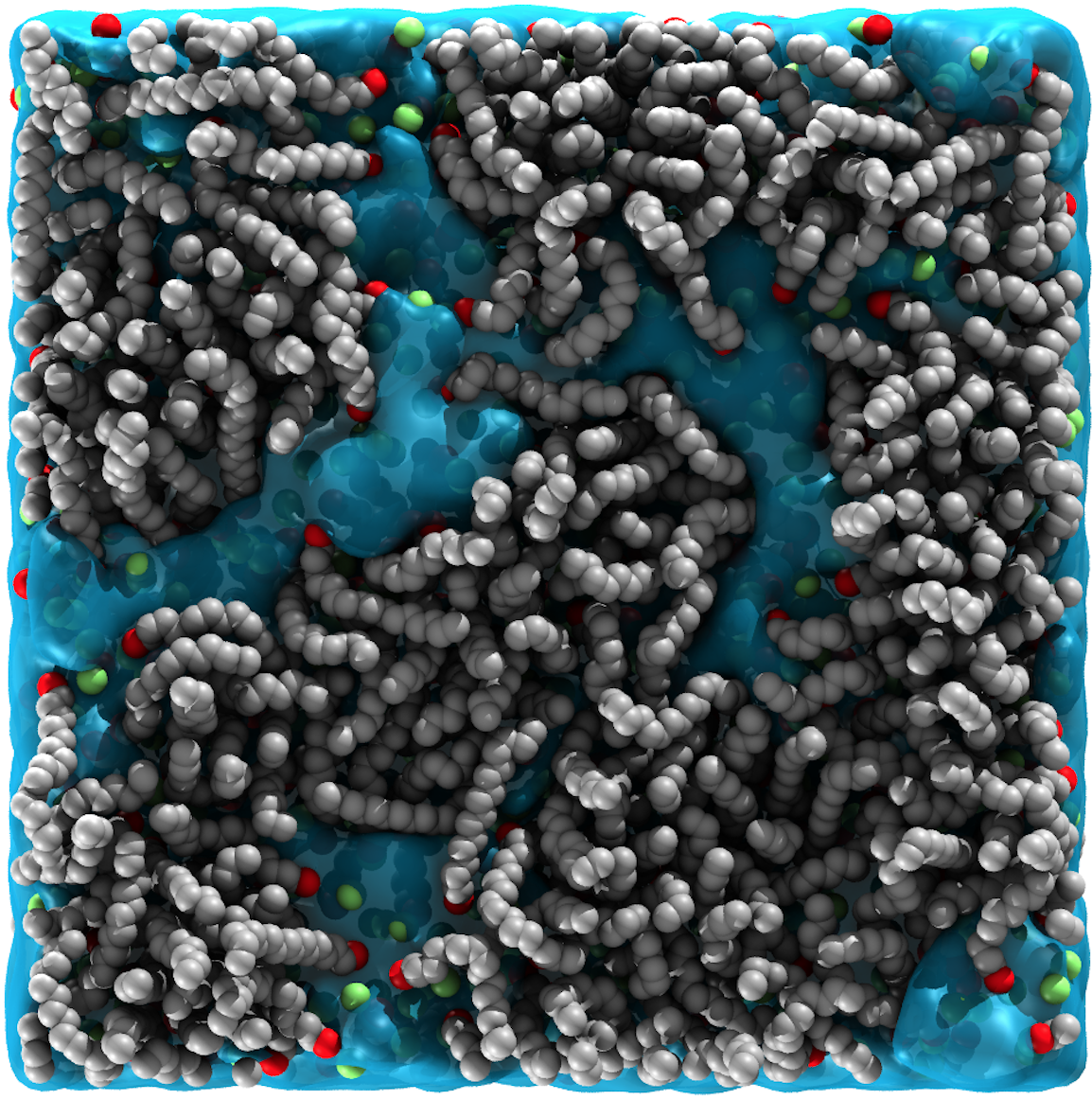
Full reference: “Structure and dynamics of nanoconfined water between surfactant monolayers“, Robert Ziolek, Franca Fraternali, Ali Dhinojwala, Mesfin Tsige & Christian D. Lorenz. Langmuir (2020)36 (1), 447 – 455.