Our recent manuscript — “Minor chemistry changes alter surface hydration to control fibronectin adsorption and assembly into nanofibrils” — reports the results of a multi-disciplinary investigation of mechanisms that govern material-driven fibrillogenesis. Previously, our collaborators in Prof. Manuel Salmeron-Sanchez‘s group at the University of Glasgow showed that fibronectin formed fibril networks when it adsorbed to poly(ethyl acrylate) substrates, but did not do so when it adsorbs to poly(methyl acrylate) (Llopis-Hernandez et al. Science Advances (2016) 2 (8), e1600188). In our recent joint manuscript, we have investigated the adsorbtion of fibronectin onto self-assembled monolayers (SAMs) which were functionalised with the ethyl acrylate and methyl acrylate side chains, which serve as model surfaces of the respective polymeric interfaces.
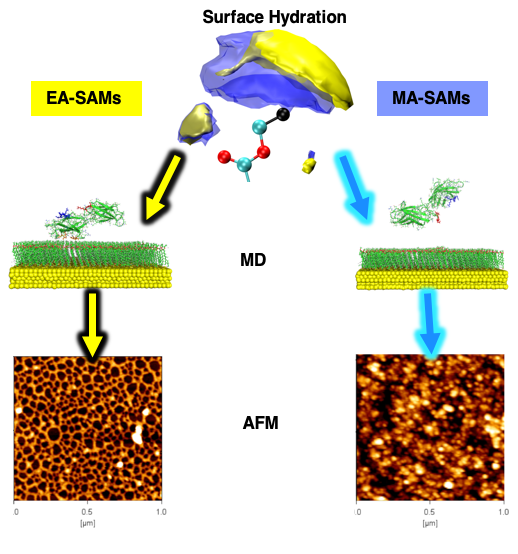 In this manuscript, Manuel’s group showed experimentally that ethyl acrylate (EA) and methyl acrylate (MA) SAMs demonstrate similar behavior in vitro to the polymers in terms of fibronectin fibrillogenesis, domain exposure, and cell adhesion. Additionally, we have carried out large-scale all-atom molecular dynamics simulations studying the interaction of the FNIII 9th and 10th domains with EA and MA SAMs, totaling four 500 ns‐long simulations. From these simulations, we observe that the 9th domain adsorbs to the EA SAMs but not to the MA SAMs, and the 10th domain does not adsorb to either interface. Here are animations of the simulated trajectories of the FNIII 9th and 10th domains interacting with the EA SAMs (http://bit.ly/2MUGnJg) and with the MA SAMs (http://bit.ly/2qVRXM1). We also find that both of the RGD and PHSRN motifs are available for cell binding when these domains adsorb to the EA SAMs. The analysis of the adsorbing residues shows that the same residue regions drive the adsorption on the EA SAMs. In the end, we show that the hydration of the MA SAMs is significantly more dense than that observed for the EA SAMs and it is this difference in the hydration layer of the SAMs interface that leads to the difference in the ability of the fibronectin to form fibrils when adsorbed.
In this manuscript, Manuel’s group showed experimentally that ethyl acrylate (EA) and methyl acrylate (MA) SAMs demonstrate similar behavior in vitro to the polymers in terms of fibronectin fibrillogenesis, domain exposure, and cell adhesion. Additionally, we have carried out large-scale all-atom molecular dynamics simulations studying the interaction of the FNIII 9th and 10th domains with EA and MA SAMs, totaling four 500 ns‐long simulations. From these simulations, we observe that the 9th domain adsorbs to the EA SAMs but not to the MA SAMs, and the 10th domain does not adsorb to either interface. Here are animations of the simulated trajectories of the FNIII 9th and 10th domains interacting with the EA SAMs (http://bit.ly/2MUGnJg) and with the MA SAMs (http://bit.ly/2qVRXM1). We also find that both of the RGD and PHSRN motifs are available for cell binding when these domains adsorb to the EA SAMs. The analysis of the adsorbing residues shows that the same residue regions drive the adsorption on the EA SAMs. In the end, we show that the hydration of the MA SAMs is significantly more dense than that observed for the EA SAMs and it is this difference in the hydration layer of the SAMs interface that leads to the difference in the ability of the fibronectin to form fibrils when adsorbed.
Full reference: “Minor chemistry changes alter surface hydration to control fibronectin adsorption and assembly into nanofibrils“, Mateusz K. Bieniek, Virginia Llopis-Hernandez, Katie Douglas, Manuel Salmeron-Sanchez & Christian D. Lorenz. Advanced Theory and Simulations (2019) doi: 10.1002/adts.201900169.