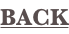Ni3Al bulk structure

6. With Lt we can now select the translational vectors
8. Use Su to choose the conventional cell which is the same as the original set, so we enter the unit matrix: 1 0 0, 0 1 0 and 0 0 1.
9. Finally, we can add the atomic positions of Ni and Al .


7. For the cubic system, you just need to specify the length of the lattice vector.
For Ni3Al this is
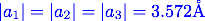


10. When you type At, tetr asks you to specify the chemical element and then the coordinates of the atom.

Al atom at 0 0 0

Ni atom at 0.5 0.5 0
The unit cell contains 4 atoms (one Al and three Ni). You need to specify the coordinates of one Ni atom only because tetr will automatically generate the others on the base of the symmetry operations of the group (see also Materials Science-Poland, Vol. 28, No. 1, 2010)
11. We are now ready to generate the unit cell with the command Gn.
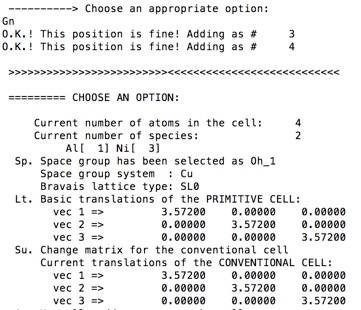
12. Type Co to see the atomic coordinates of the unit cell
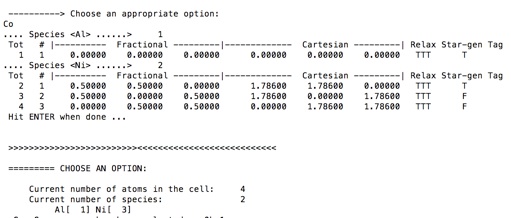
The unit cell generated contains 4 atoms : Al (0 0 0 ) , Ni (1/2 1/2 0), Ni (1/2 0 1/2) and Ni (0 1/2 1/2).
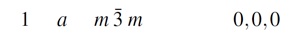
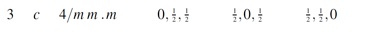
From the International Crystallographic tables ((Vol.A, 2002)) we can check the positions of the atoms:
Al position
Ni positions